Ethics, Compliance and Regulatory Guidance
CenterWatch | Insights
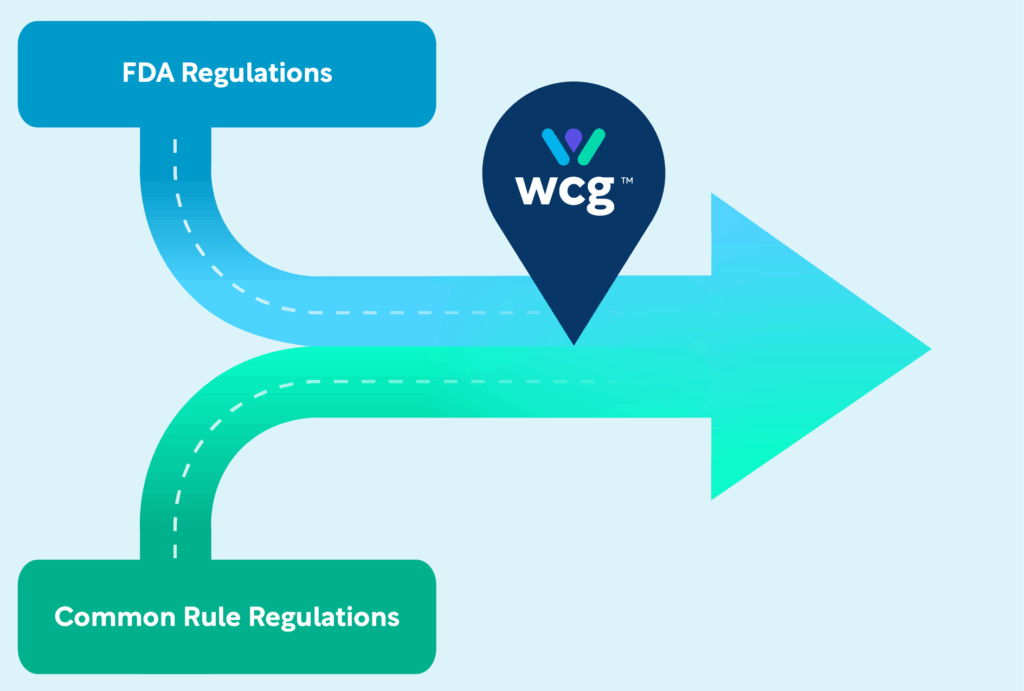
How WCG Is Preparing for FDA Harmonization with the Common Rule
Blog Posts
CenterWatch | Insights
The Ethical and Safety Considerations of Accelerating Oncology Trials
Podcasts
CenterWatch | Insights
Ask the Experts: What is the difference between a Risk Group and a Biosafety Level?
Blog Posts
CenterWatch | Insights
What Is Assent, When Provided by an Adult Participant Lacking Capacity? And What Are WCG’s Expectations and the Investigator’s Responsibilities?
Blog Posts
CenterWatch | Insights
A Review Of The Regulatory Landscape for In Vitro Gametogenesis
Blog Posts
CenterWatch | Insights
If My Study Includes Approved Drugs, When Do the Risks of Those Drugs Need to Be Disclosed in the Consent Form?
Blog Posts
CenterWatch | Insights
Myth vs Reality: Understanding Suicidality Risk in All Clinical Trials
Blog Posts
CenterWatch | Insights
FDA’s Path Toward Diversity in Clinical Trials: The DEPICT Act and Sponsor Responsibility
Whitepapers
CenterWatch | Insights
Delve into the World of Psychedelic Research and Ethical Inquiry
Podcasts
CenterWatch | Insights