August 2003 – The CenterWatch Monthly : PDF
$79.00
Product Details
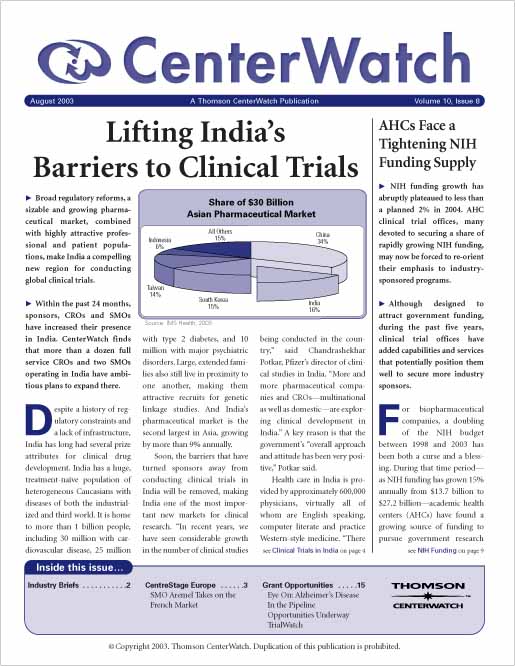
Lifting India’s Barriers to Global Clinical Trials
Broad regulatory reforms, a sizable and growing pharmaceutical market, combined with highly attractive professional and patient populations, make India a compelling new region for conducting clinical trials. Within the past 24 months, sponsors, CROs and SMOs have increased their presence in India. CenterWatch estimates that more than a dozen full service CROs and two SMOs operating in India have ambitious plans to expand there.
AHCs Face a Tightening NIH Funding Supply
NIH funding growth has abruptly plateaued to less than a planned 2% in 2004. AHC clinical trial offices, many devoted to securing a share of rapidly growing NIH funding, may now be forced to re-orient their emphasis to industry-sponsored programs.
Also in this issue:
- SMO Aremel Takes on the French Market
- Eye on: Alzheimer's