August 2016 – The CenterWatch Monthly : PDF
$79.00
Product Details
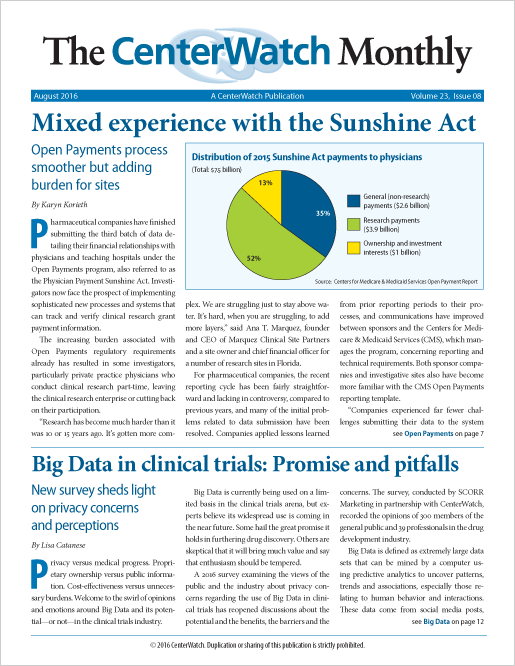
Mixed experience with the Sunshine Act
Pharmaceutical companies have finished submitting the third batch of data detailing their financial relationships with physicians and teaching hospitals under the Open Payments program, also referred to as the Physician Payment Sunshine Act.
Big Data in clinical trials: Promise and pitfalls
Privacy versus medical progress. Proprietary ownership versus public information. Cost-effectiveness versus unnecessary burdens. Welcome to the swirl of opinions and emotions around Big Data and its potential—or not—in the clinical trials industry.
Also in this issue:
- To treat rare diseases, every patient counts
- The importance of multifaceted virtual investigator meetings
- Regulatory Update
- Month in Review
- FDA Actions
- Study Lead Opportunities
- New Drugs in the Pipeline