November 2011 – The CenterWatch Monthly : PDF
Product Details
CRAs see role evolving into quality, compliance, start-up
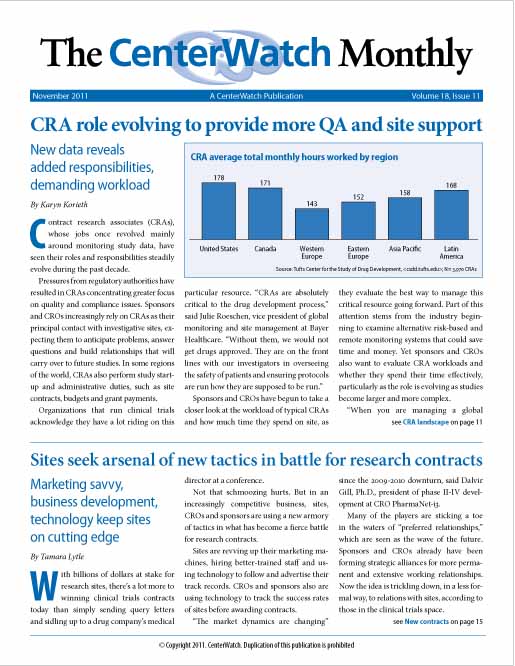
Contract research associates (CRAs), whose jobs once revolved mainly around monitoring study data, have seen their roles and responsibilities steadily evolve during the past decade. Regulatory pressures have resulted in CRAs focusing more on quality and compliance issues. Sponsors and CROs increasingly rely on CRAs as their principal contact with sites, anticipating problems, answering questions and building relationships. In some regions, CRAs also perform study start-up and administrative duties. Sponsors and CROs have begun to take a closer look at the workload of typical CRAs. Until now, the industry has lacked data about the...
Sites seek arsenal of new tactics in battle for research contracts
With billions of dollars at stake for research sites, there’s a lot more to winning clinical trials contracts than simply sending query letters and sidling up to a drug company’s medical director at a conference. In an increasingly competitive business, sites, CROs and sponsors are using a new armory of tactics in what has become a fierce battle for research contracts. Sites are revving up their marketing machines, hiring better-trained staff and using technology to follow and advertise their track records. The strategic alliances sponsors and CROs have been forming are now...
Eye On MedImmune
MedImmune attributes its strength in the biotechnology sector to discovery, research optimization, process development, entrepreneurial spirit and its commitment to developing new biologics. It is considered to be an industry leader in the manufacture of monoclonal antibodies and live viral vaccines. In hopes of reducing development costs, MedImmune is considering various options to lower development cycle times and...
- Unlocking South East Asia for patient recruitment
- Quality oversight in every clinical trial
- Industry Briefs
- The Pulse on Recruitment
- TrialWatch
- New Study Launches