Blog Posts

CenterWatch | Insights
Beyond One-and-Done: Exploring Redosing Strategies in Gene Therapy
Blog Posts
CenterWatch | Insights
Breakthroughs in In Vivo Gene Editing: Insights from ASGCT’s 2025 Conference
Blog Posts
CenterWatch | Insights
Beyond Binaries: Gender-Inclusive Language in Clinical Research
Blog Posts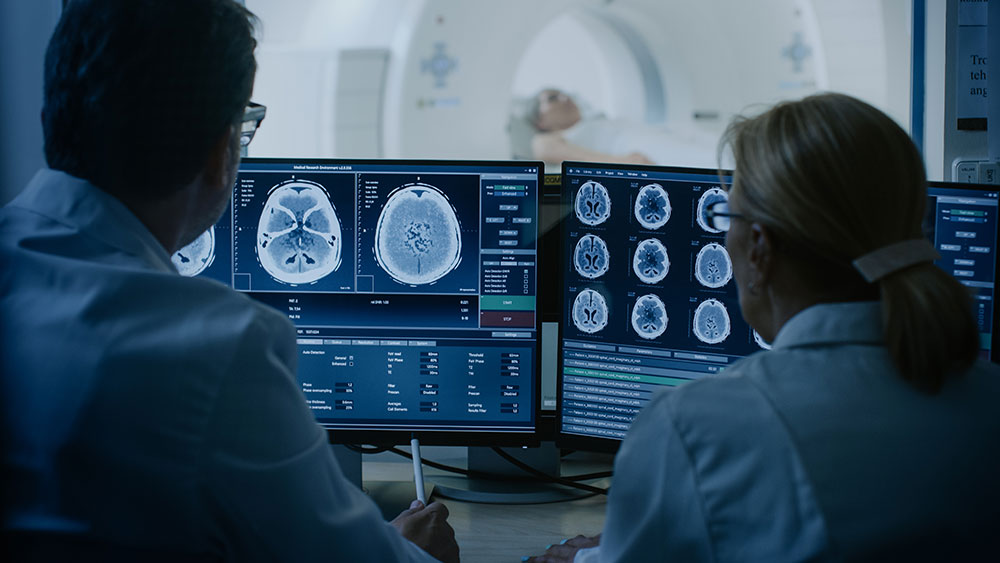
CenterWatch | Insights
FDA Draft Guidance on Multiregional Clinical Development Programs for Oncology
Blog Posts
CenterWatch | Insights
Ensuring Representative US Enrollment in Oncology Clinical Trials: Navigating the Rising Tide of Regulatory Scrutiny
Blog Posts
CenterWatch | Insights
Eyewash Stations & Gene Transfer Studies: NIH Guidelines Explained
Blog Posts
CenterWatch | Insights
How Long Should Eyes Be Flushed After Exposure?
Blog Posts
CenterWatch | Insights
FDA Guidance on AI-Enabled Devices: Transparency, Bias, & Lifecycle Oversight
Blog Posts
CenterWatch | Insights
Regulatory Evaluations of Companion Diagnostic Medical Devices in Clinical Trials: Experiences of an Independent IRB
Blog Posts
CenterWatch | Insights